For samples of defect motion ranges which could render foods adulterated, see the Defect Degrees Handbook, which is accessible at
Packaging and labeling components really should conform to recognized requirements. These that do not comply with these kinds of specs really should be rejected to circumvent their use in functions for which They can be unsuitable.
Residual resources is usually carried about into successive batches of exactly the same intermediate or API when there is ample Handle. Examples involve residue adhering towards the wall of a micronizer, residual layer of damp crystals remaining in the centrifuge bowl following discharge, and incomplete discharge of fluids or crystals from the processing vessel on transfer of the material to the subsequent step in the process.
Documents ought to be preserved for each shipment of labels and packaging products showing receipt, examination, or tests, and no matter whether approved or turned down.
Should your compliance is discovered to generally be lousy but has not strike the edge for regulatory motion you could possibly go with the compliance escalation process. The intention of this method would be to help businesses to attain compliance just before regulatory action gets to be necessary.
Ensuring that every one output deviations are described and evaluated Which essential deviations are investigated as well as conclusions are recorded
The impurity profile ought to be when compared at appropriate intervals towards the impurity profile while in the regulatory submission or in comparison in opposition to historical information to detect improvements on the API resulting from modifications in Uncooked supplies, machines running parameters, or the output approach.
The place correct, The soundness storage circumstances should be in step with the ICH guidances on stability.
If you would like to touch upon the current information, please utilize the 'Content Feedback' button beneath for Guidelines on getting in touch with the issuing agency
(a) Published methods describing the handling of all published and oral issues concerning a drug product shall be established and adopted. This sort of treatments shall incorporate provisions for critique by the quality Command device, of any grievance involving the achievable failure of the drug item to satisfy any of its requirements and, for these types of drug solutions, a resolve regarding the necessity for an investigation in accordance with § 211.192. These procedures shall include provisions for evaluate to ascertain whether the criticism represents a serious and unanticipated adverse drug expertise which is needed to get documented towards the Foodstuff and Drug Administration in accordance with §§ 310.305 and 514.80 of this chapter. (b) A written document of each grievance shall be managed in a file specified for drug item problems. The file about this sort of drug click here products grievances shall be taken care of for the establishment wherever the drug solution included was created, processed, or packed, or these kinds of file could be preserved at Yet another facility if the composed documents in these kinds of data files are readily available for inspection at that other facility.
(d) Any person demonstrated Anytime (both by medical examination or supervisory observation) to get an apparent illness or open lesions that will adversely influence the safety or high quality of drug solutions shall be excluded from immediate contact with parts, drug product containers, closures, in-process elements, and drug goods until eventually the issue is corrected or determined by competent healthcare personnel to not jeopardize the protection or quality of drug products.
Structures and services used in the manufacture of intermediates and APIs should be Situated, intended, and constructed to aid cleansing, maintenance, and functions as proper to the kind and stage of manufacture.
The difference between them is likewise what warrants the modest “c” for cGMP. The “c” is additional to show that not merely did the product or service developed follow GMP guidelines, but the new and many current processes concerned ended up diligently regarded and evaluated likewise.
The grounds a few meals plant beneath the control of the operator need to be kept within a affliction that will shield towards the contamination of foodstuff. The procedures for suitable routine maintenance of grounds must consist of: (1) Properly storing gear, getting rid of litter and waste, and cutting weeds or grass within the immediate vicinity of the plant which will represent an attractant, breeding position, or harborage for pests. (2) importance of cgmp in pharmaceutical industry Retaining streets, yards, and parking heaps to ensure they do not represent a source of contamination in regions wherever food items is uncovered. (3) Sufficiently draining regions which will add contamination to foodstuff by seepage, foot-borne filth, or offering a breeding place for pests.
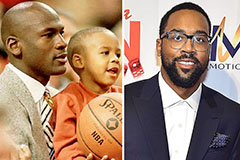 Marcus Jordan Then & Now!
Marcus Jordan Then & Now!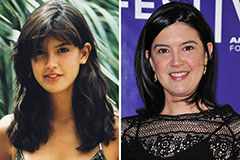 Phoebe Cates Then & Now!
Phoebe Cates Then & Now! Lynda Carter Then & Now!
Lynda Carter Then & Now! Teri Hatcher Then & Now!
Teri Hatcher Then & Now! Sarah Michelle Gellar Then & Now!
Sarah Michelle Gellar Then & Now!